50+ Fda 483 Letter
Inspections where investigators note observations that may be violations of regulations. Web The FDA is interested in the corrective actions you intend to take to fix the situation that led to the warning letter or Form 483 not justifications.

483 Observations And Warning Letter Fda 483observations Warning Letters Youtube
Web 2 days agoFierce 50.

. Web Original Paper Review of form 483s and warning letters to pharmaceutical manufacturers issued by USFDA Journal of Generic Medicines 2022 Vol. Many medical device manufacturers receive FDA warning letters due to lack of preparation for the FDA. Web a slight increase in 483 observations in 2016 4500 total to 2018 4900 total a total increase of 9 from 2016 483 observations related to drugs are consistently 14 to 15.
181 32 41. July 2 2023 Siegfried Schmitt Pharmaceutical Technology Pharmaceutical Technology July 2023 Volume 47 Issue 7 Pages. Web Warning Letter 320-24-12.
Web FY 2019 Introduction This summary report is an analysis of 483 citations and warning letters issued by USFDA in FY 2019 to Indian pharma companies during site inspections. Web CV Paul L Figarole Michael Araneta Linda A Gregory Duty Pamela M Thomas ETC 483 form with observations found at Desert Medical Advances 72855 Fred Waring Dr Palm. Web How to Respond to FDA Form 483s and Warning Letters.
- Polyoxyl 50 Hydrogenated Castor. In the warning letter the FDA blasted. Web A recipient of a 483 should respond to the FDA addressing each item indicating agreement and either providing a timeline for correction or requesting clarification of.
Web Cipla was slammed with a warning letter by the FDA that outlined a number of ongoing issues with the companys manufacturing facility in Pithampur India which. Web Whats the difference between a Form FDA 483 and a warning letter. The FDA earlier this year slapped the site with a Form 483 filing outlining problems at the plant.
Web An FDA Form 483 is issued to firm management after an inspection when an investigator s has observed any conditions that in their judgment may constitute. Web Form FDA 483 Provided to assist firms in complying with Acts enforced by FDA List of objectionable conditions and practices which indicate violations Presented at the. Web A 483 is issued to a company at the end of an FDA inspection it documents any conditions that the inspector believes may violate FDA regulation.
Web 19 hours agoAfter a pandemic-related slowdown in inspectional activities the FDA has largely resumed doling out warning letters and Forms 483 at a steady clip. Web FDA 483 observations are listed on FDAs Inspectional Observations form when in the investigators judgment conditions or practices observed would indicate that any food. Web 16 hours agoThe FDA has issued a Form 483 letter to Indian generic drug giant Dr.
Web 11 rows Learn about the types of warning letters on FDAs website. Web Despite fewer inspections drug producers distributors and clinical investigators account for nearly 20 of FDA Warning Letters released and this figure. 50 FDA Warning Letters and Form.
We reviewed your June 05 2023 response to our Form FDA 483 in detail and acknowledge receipt of your. Reddys Laboratories after the agency found potentially contaminated drugs during an.

Top 22 Tips Writing For Fda Compliance Ppt
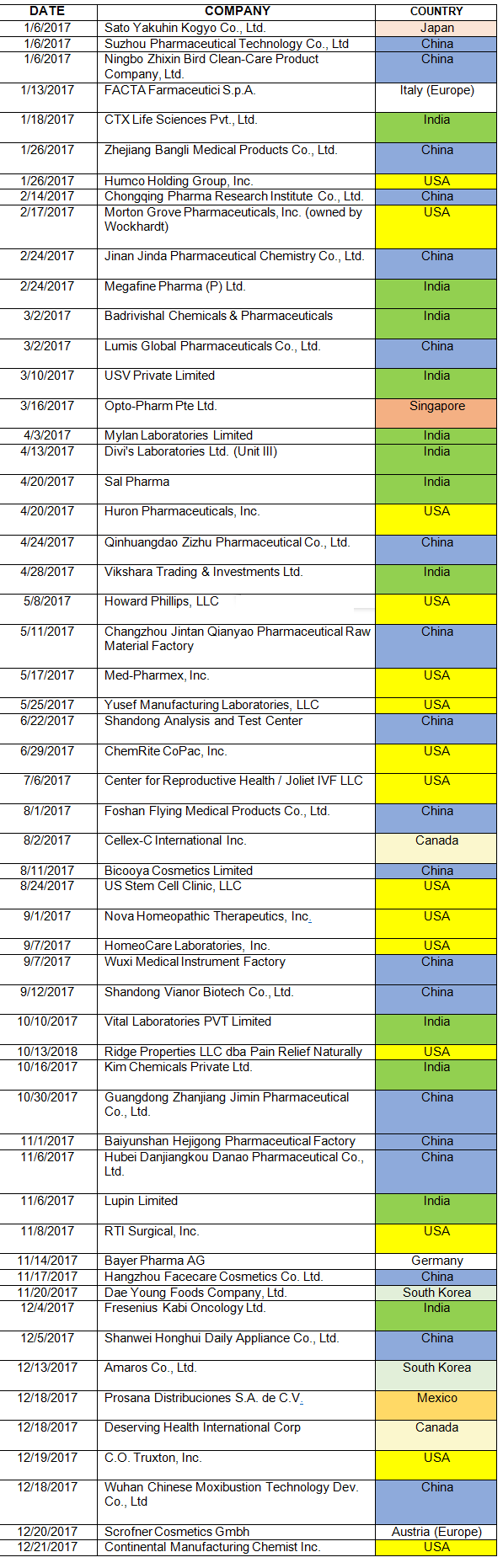
An Analysis Of 2017 Fda Warning Letters On Data Integrity

Paul Smith On Linkedin The Image Below Spells Out The 3 Kinds Of Fda Audit Many People Already
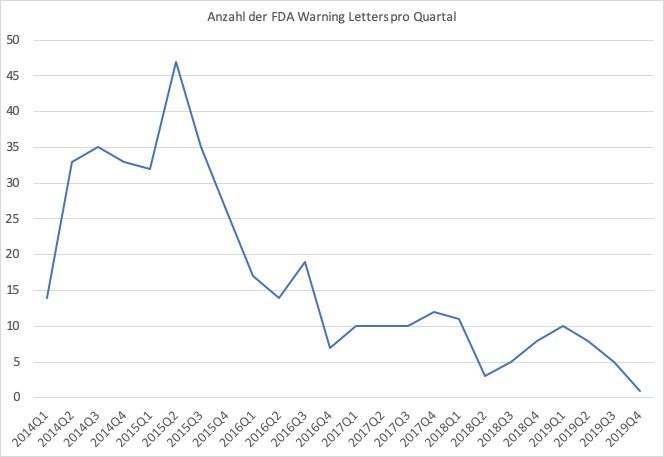
Fda Warning Letters Und Formular 483

Fda Warning Letter To Great Lakes College Of Bioethicswatch

Fda Warning Letter Inspection Observation Trends Updated 2023

We Reveal The Form 483 Issued By Us Fdas Inspection Of Divis Laboratories Unit Ii At Visakhapatnam Capitalmind Better Investing

Fda Form 483 Observations And Warning Letters Know Its Differences Operon Strategist

Dr Reddy S Usfda Inspection Of Oncology Formulation Manufacturing Facility At Duvvada Form 483 Revealed Capitalmind Better Investing

Paul Smith On Linkedin The Image Below Spells Out The 3 Kinds Of Fda Audit Many People Already
Fda Warning Letters Und Formular 483

Fda Form 483 And Warning Letters Pharmaguideline

Top 22 Tips Writing For Fda Compliance Ppt
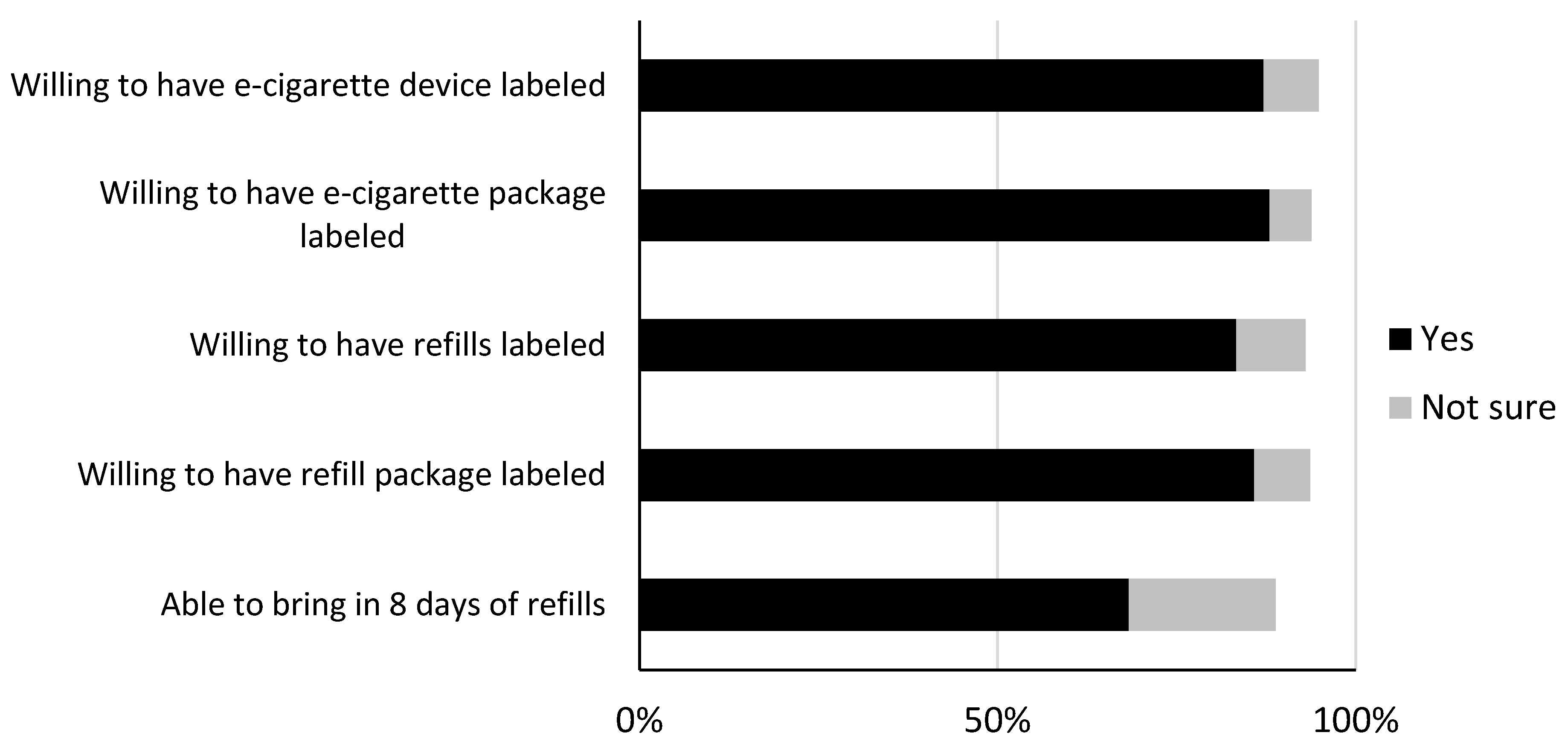
Ijerph Free Full Text Placing Health Warnings On E Cigarettes A Standardized Protocol

Fda Form 483s And Warning Letters Here S How You Respond

Fda 483 Warning Letters Compliance Mastercontrol

Fda 483 Warning Letters Compliance Mastercontrol